Introduction: Idecabtagene vicleucel (ide-cel, bb2121) is a B-cell maturation antigen-directed chimeric antigen receptor (CAR) T cell therapy under investigation in the KarMMa trial as a treatment for patients with relapsed and refractory multiple myeloma (RRMM) who are triple-class exposed to immunomodulatory drugs, proteasome inhibitors, and anti-CD38 antibodies. In the phase 2 KarMMa trial, ide-cel demonstrated a favorable benefit-risk profile in this patient population (Munshi NC, et al. J Clin Oncol 2020;38:8503). Ide-cel also demonstrated clinically meaningful improvements in the key health-related quality of life (HRQoL) symptoms associated with MM (Delforge M, et al. HemaSphere 2020;4:EP1000). Translating HRQoL data to health state utility values (HSUVs)/HRQoL weights is key to understanding the HRQoL impact of a treatment in relation to that of a healthy general population and is an important consideration in clinical decision making and health technology assessments. HSUVs are scored between 0 and 1, where 0 is death and 1 is perfect health. In the general population, individuals of a similar age range to patients with MM have HSUV scores of 0.83 in the USA, 0.80 in the UK, and 0.84 in Canada (Guertin JR, et al. CMAJ 2018;190:E155-161; Janssen MF, et al. Eur J Health Econ 2019;20:205-216). This analysis aimed to determine HSUVs for patients treated in the KarMMa study according to their progression status.
Methods: HRQoL assessment in the KarMMa study (NCT03361748) included the European Quality of Life-5 dimensions 5 levels (EQ-5D-5L) health state classifier performed at specified time points: prior to receiving lymphodepleting chemotherapy (baseline), day of infusion (Day 1), Months 1, 2, 3, 6, 9, 12, and 15, inclusive of disease progression/relapse or complete remission. Using US, UK, and Canadian weights, HSUVs were estimated for the KarMMa trial at an aggregate level. A longitudinal mixed-effects model was used with health state as a fixed effect, and a random intercept term. Three models were run using different health states. Model 1 considered 3 health states: baseline, pre-progression, and post-progression. In Model 2, the pre-progression health state was split into 2 time periods: Day 1 to the end of Month 1, and Month 2 onward. In Model 3, 2 pre-progression health states were defined based on the quality of response to treatment, thus capturing the difference in HRQoL for patients achieving at least a very good partial response (≥ VGPR) or patients who did not achieve a VGPR (< VGPR).
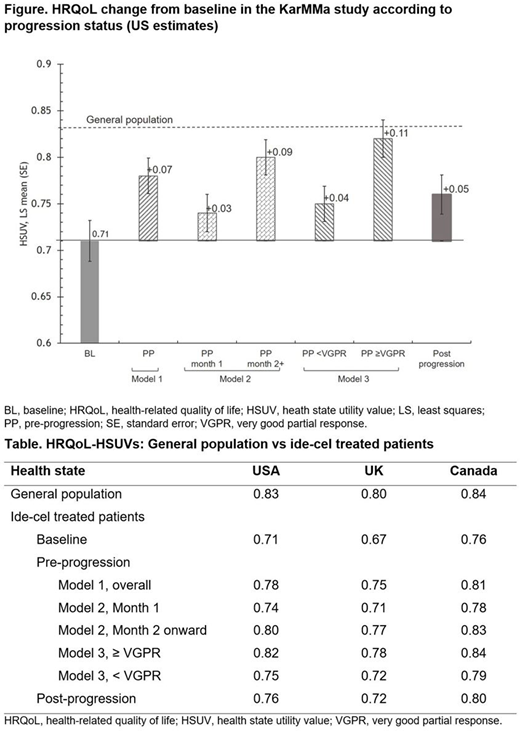
Results: The HSUVs derived from the 3 different models using US, UK, and Canadian tariffs are summarized (Table). In all 3 models, patients in the pre-progression state experienced an increase in HRQoL from baseline. In Model 1, the increment ranged from +0.05 to +0.08. On progression, patients experienced a decrement (−0.01 to −0.03), but their HSUV remained above the baseline value by +0.04 to +0.05, indicating that ide-cel treatment was associated with an improvement in HRQoL, with some of the benefit remaining even upon disease progression. When HSUV in the pre-progression state was analyzed in Model 2 at Month 1 and then Month 2 onward, patients also experienced an increase in their HRQoL from baseline. While this increase was small in Month 1 (+0.02 to +0.04), the subsequent increase from baseline (i.e. from Month 2 onward) was more pronounced (+0.07 to +0.10) reflecting the benefits of a one-off administration of ide-cel and the associated treatment-free interval. A further analysis of the pre-progression HSUV by the quality of response in Model 3 showed that patients who achieved ≥ VGPR had a greater improvement in HRQoL (+0.08 to +0.11) than patients who achieved < VGPR. Both response levels were associated with an improvement in HRQoL compared with baseline, with HRQoL for patients achieving ≥ VGPR approaching that of the general population (Figure).
Conclusions: Results of this analysis indicate that ide-cel provides clinically meaningful improvements in HRQoL for patients with triple-class-exposed RRMM. The benefit is particularly marked in patients who achieve ≥ VGPR, in whom HRQoL approaches that of the general population.
Delforge:Janssen: Honoraria, Research Funding; BMS: Honoraria, Research Funding; Takeda: Honoraria; Amgen: Honoraria. Shah:BMS, Janssen, Bluebird Bio, Sutro Biopharma, Teneobio, Poseida, Nektar: Research Funding; GSK, Amgen, Indapta Therapeutics, Sanofi, BMS, CareDx, Kite, Karyopharm: Consultancy. Rodriguez-Otero:Celgene-BMS: Consultancy, Honoraria; Mundipharma: Research Funding; Janssen, BMS: Other: Travel, accommodations, expenses; BMS, Janssen, Amgen: Honoraria; Janssen, BMS, AbbVie, Sanofi, GSK, Oncopeptides, Kite, Amgen: Consultancy, Honoraria. Hari:Amgen: Consultancy; GSK: Consultancy; Janssen: Consultancy; BMS: Consultancy; Incyte Corporation: Consultancy; Takeda: Consultancy. Braverman:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Trigg:Adelphi Values: Current Employment. Patel:BMS: Current Employment. Huang:BMS: Current Employment, Current equity holder in publicly-traded company. Hege:Arcus Biosciences: Divested equity in a private or publicly-traded company in the past 24 months; Mersana Therapeutics: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; BMS: Current Employment, Current equity holder in publicly-traded company, Other: Travel, accommodations, expenses, Patents & Royalties: Numerous, Research Funding. Dhanasiri:BMS: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal